A New Comprehensive Model of AMD: The AMD-11 Framework and the Modifiable Pathways That Shape Macular Health
Age-Related Macular Degeneration (AMD) is one of the leading causes of vision impairment in older adults. Yet despite decades of research, AMD is still widely misunderstood. Many people believe that AMD is caused by “aging alone,” but the scientific reality is far more complex — and far more hopeful.
AMD develops slowly through multiple interacting biological pathways, not a single trigger. These pathways affect how the retina uses energy, handles oxidative stress, clears waste, manages lipids, receives blood flow, and responds to inflammation. Genetics and lifestyle also play a significant role.
To help make this complexity clearer and more useful for patients, families, and clinicians, we developed a new model:
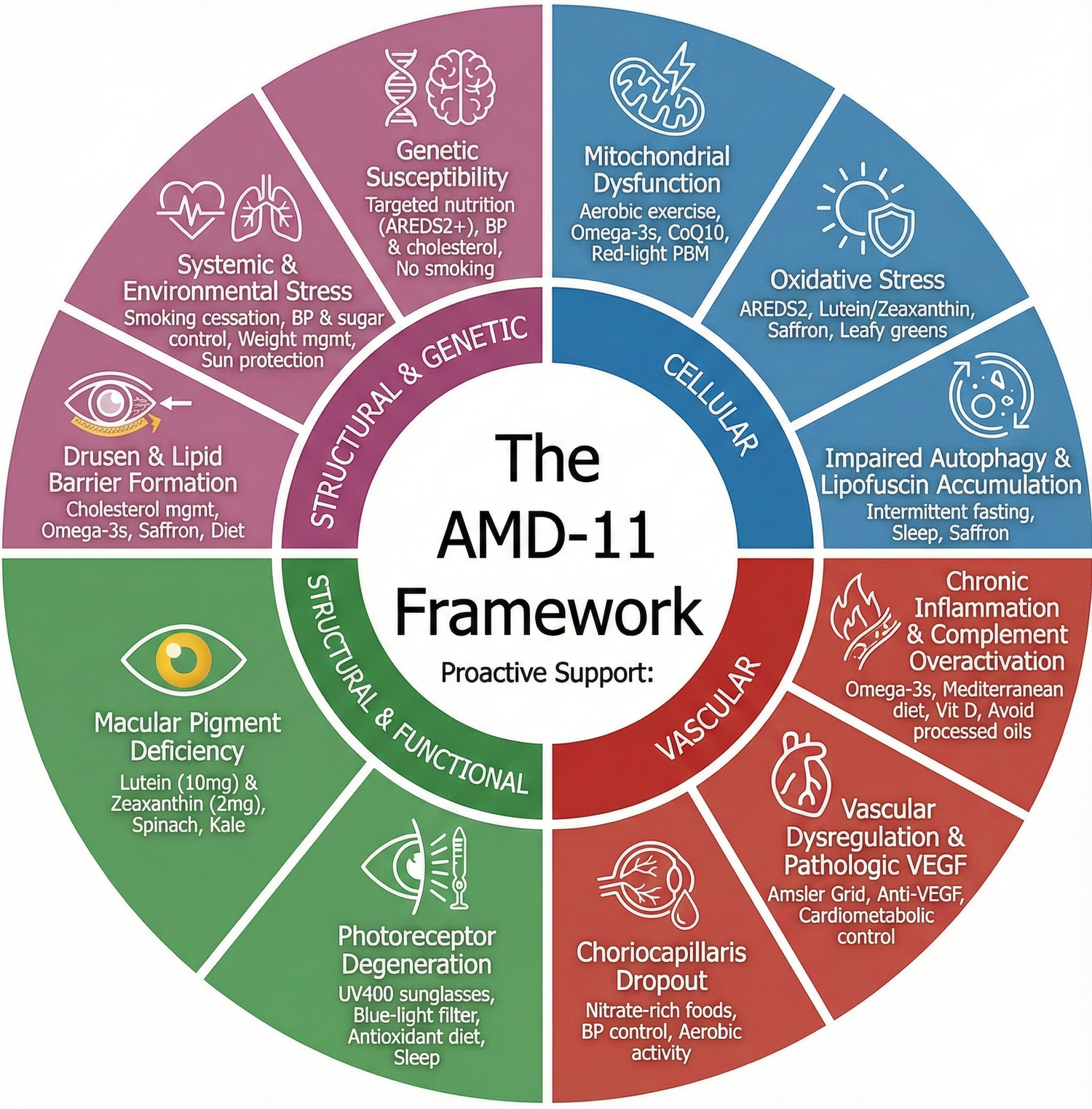
🌟 The AMD-11 Framework
A unified way to understand eleven major mechanisms that drive AMD — and which of these mechanisms can be influenced through lifestyle, nutrition, monitoring, and healthy habits.
The AMD-11 Framework organizes these mechanisms into four categories:
- Cellular Mechanisms
- Vascular Mechanisms
- Structural & Functional Mechanisms
- Systemic & Genetic Influences
Below, we explore all 11 mechanisms — and the research-supported areas where proactive steps may help support long-term macular health.
I. Cellular Mechanisms (The Retina’s Internal Engine)
1. Oxidative Stress
The retina is one of the most metabolically active tissues in the body, constantly exposed to light and oxygen. This produces reactive oxygen species (ROS), the “exhaust fumes” of cellular activity.
With age and disease, the retina’s antioxidant defenses weaken, allowing oxidative damage to accumulate.
Why it matters:
Chronic oxidative stress is a central feature of AMD and accelerates injury to the retinal pigment epithelium (RPE) and photoreceptors.
Supportive strategies:
• Nutrients from the AREDS2 formula
• Diet rich in leafy greens and antioxidants
• Research-backed botanical compounds like saffron, which have been studied for their role in balancing oxidative stress and supporting retinal function
2. Mitochondrial Decline
RPE cells require enormous amounts of energy. Over time, their mitochondria — the cell’s power generators — become less efficient.
Why it matters:
When mitochondrial energy output drops, retinal cells become more fragile and less able to maintain normal function.
Supportive strategies:
• Regular aerobic exercise
• Balanced nutrition
• Investigational modalities such as red-light (PBM) therapy
• Research suggests saffron compounds may support mitochondrial resilience and signaling pathways
3. Chronic Inflammation & Complement Activation
The immune system plays a major role in AMD. When complement proteins are over-activated, the retina becomes a target of chronic, low-grade inflammation.
Why it matters:
This persistent inflammation damages RPE cells and contributes to drusen formation and progression to advanced AMD.
Supportive strategies:
• Omega-3 fatty acids (EPA/DHA)
• Reduced intake of processed oils
• Anti-inflammatory dietary patterns
4. Impaired Autophagy & Lipofuscin Buildup
Healthy cells routinely recycle waste and damaged components (autophagy). In AMD, this process slows, allowing toxic lipofuscin granules to accumulate.
Why it matters:
Lipofuscin buildup stresses the RPE and interferes with waste clearance.
Supportive strategies:
• High-quality sleep (linked to retinal detox pathways)
• Structured overnight fasting windows (12–14 hrs), which are associated with autophagy activation
• Reducing oxidative burden to prevent further waste accumulation
II. Vascular Mechanisms (Blood Flow & Oxygen Delivery)
5. Choriocapillaris Dropout
The choriocapillaris — a dense layer of tiny blood vessels — begins thinning early in AMD.
Why it matters:
Reduced blood flow limits oxygen and nutrients reaching the retina.
Supportive strategies:
• Diets rich in natural nitrates (beets, spinach, arugula) to support nitric oxide and microvascular health
• Regular physical activity
• Managing cardiovascular health
6. VEGF-Driven Neovascular Stress (Wet AMD)
When the retina becomes oxygen-starved, it releases VEGF to grow new blood vessels. These vessels are fragile, causing leakage and scarring.
Why it matters:
Wet AMD can cause sudden vision changes if not treated quickly.
Supportive strategies:
• Regular monitoring with an Amsler grid
• Prompt consultation if distortion appears
• Anti-VEGF injections (clinical treatment)
III. Structural & Functional Mechanisms
7. Drusen & Lipid Accumulation
Drusen are lipid- and protein-rich deposits that form between the RPE and Bruch’s membrane.
Why it matters:
They disrupt nutrient flow, trap waste, and activate immune pathways.
Supportive strategies:
• Managing blood lipids and cholesterol
• Heart-healthy eating patterns
• Consistent follow-up with an eye-care professional
8. Macular Pigment Depletion
Lutein and zeaxanthin form a natural yellow “protective filter” over the macula.
Why it matters:
Low pigment density reduces protection against blue light and oxidative stress.
Supportive strategies:
• Lutein + zeaxanthin supplementation
• Foods such as spinach, kale, and yellow/orange vegetables
• Blue-light filtering eyewear
9. Photoreceptor Stress & Degeneration
Photoreceptors depend entirely on RPE cells for nutrition and waste removal. When the RPE is compromised, photoreceptors lose their support.
Why it matters:
This leads to blind spots (scotomas) and decreased visual performance.
Supportive strategies:
• Consistent UV/blue-light protection
• Healthy sleep patterns
• Nutrients shown to support photoreceptor responses, including saffron (based on clinical studies)
IV. Systemic & Genetic Influences
10. Systemic & Epigenetic Stress
Lifestyle factors can accelerate every other AMD mechanism.
Why it matters:
Smoking, hypertension, poor diet, and high blood sugar are among the strongest modifiable risk factors.
Supportive strategies:
• Quit smoking
• Manage blood pressure and glucose
• Maintain a nutrient-rich diet
11. Genetic Susceptibility (CFH, ARMS2, APOE)
Genetics influence inflammation, lipid handling, and complement activity.
Why it matters:
Genes “load the gun,” but lifestyle often “pulls the trigger.”
Supportive strategies:
• Healthy habits
• Omega-3 intake
• Maintaining antioxidant and pigment levels
• Supporting mitochondrial and inflammatory balance
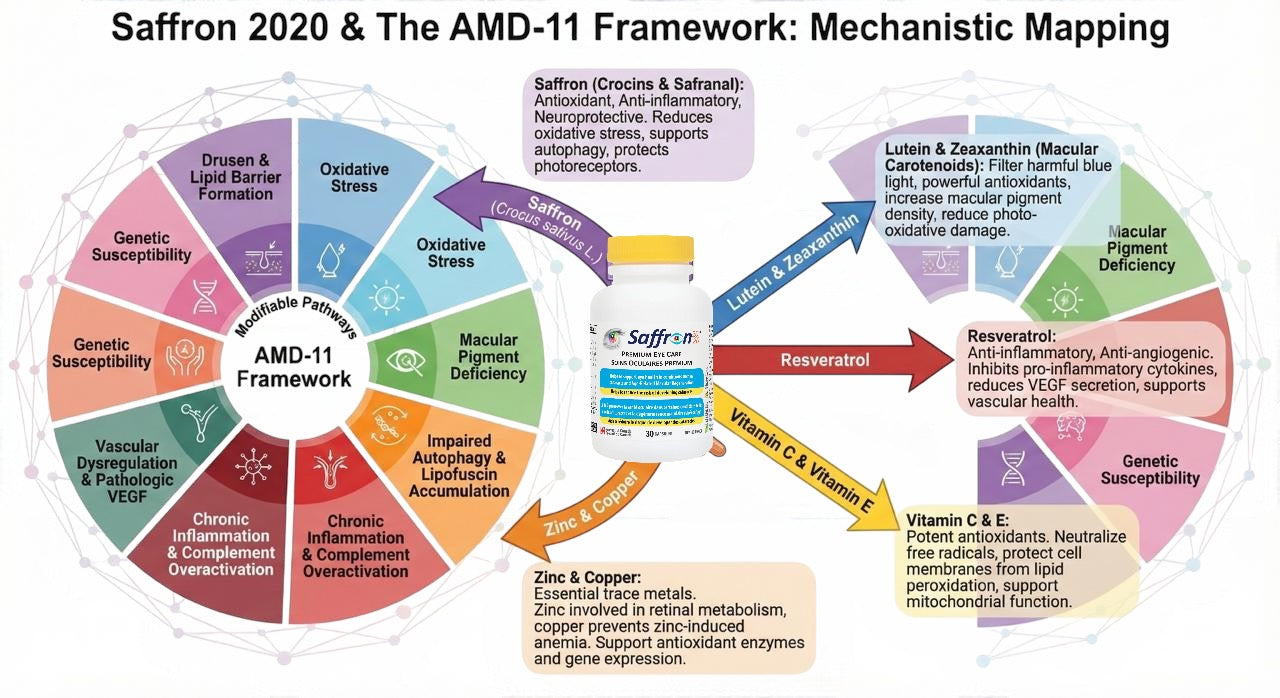
Why Saffron Has Become an Ingredient of Interest
Saffron continues to attract scientific attention because its bioactive compounds have been studied in relation to several AMD pathways, including:
- Supporting retinal function and signaling
- Modulating oxidative stress
- Influencing mitochondrial resilience
- Helping protect photoreceptor responses
These findings make saffron uniquely relevant in a multi-pathway model like the AMD-11 Framework.
A Clearer Picture for Patients and Families
The AMD-11 Framework is designed to help people understand:
- Why AMD progresses
- Where lifestyle and nutrition may make a difference
- Which areas deserve long-term monitoring
- How multiple mechanisms interact within the retina
To learn how special supplement Saffron 2020 can help preserve your vision and macular health within the AMD-11 Framework read the in-depth article by clicking here.....
References:
1. Oxidative Stress & Mitochondrial Dysfunction (Cellular)
- Mimura T. Oxidative Stress in Age-Related Macular Degeneration. 2025.
https://pmc.ncbi.nlm.nih.gov/articles/PMC12561695/ PMC - Qu S et al. Age-Related Macular Degeneration and Mitochondria-Targeted Therapies. Int J Mol Sci. 2024.
https://www.mdpi.com/1422-0067/25/3/1624 MDPI - Tong Y et al. Role of Mitochondria in Retinal Pigment Epithelial Aging and AMD. Front Aging. 2022.
https://www.frontiersin.org/articles/10.3389/fragi.2022.926627/full Frontiers - Davinelli S et al. Sleep and Oxidative Stress: Current Perspectives on the Role of Sleep in Redox Homeostasis.Antioxidants. 2024.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11199221/ PMC - Vaccaro A et al. Sleep Loss Can Cause Death through Accumulation of Reactive Oxygen Species in the Gut. Cell. 2020.
https://www.cell.com/cell/fulltext/S0092-8674(20)30555-9 Cell
2. Autophagy, Lipofuscin & Cellular “Waste” Clearance
- Zhang Z et al. Autophagy in Dry AMD: A Promising Therapeutic Strategy for Retinal Degeneration. Prog Retin Eye Res. 2024.
https://pubmed.ncbi.nlm.nih.gov/38593971/ PubMed - Kaarniranta K et al. Autophagy in Age-Related Macular Degeneration. Autophagy. 2023.
https://www.tandfonline.com/doi/full/10.1080/15548627.2022.2069437 Taylor & Francis Online - Intartaglia D et al. Autophagy in the Retinal Pigment Epithelium: A New Vision and Future Challenges. FEBS J. 2022.
https://febs.onlinelibrary.wiley.com/doi/10.1111/febs.16018 FEBS Journal - Shahhossein-Dastjerdi S et al. Autophagy and Exocytosis of Lipofuscin in Human Retinal Pigment Epithelium.IOVS. 2024.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11044829/ PMC - Si Z et al. The Role of Retinal Pigment Epithelial Cells in AMD: Phagocytosis and Autophagy. Biomolecules. 2023.
https://www.mdpi.com/2218-273X/13/6/901 MDPI
3. Chronic Inflammation & Complement Overactivation
- Deng Y et al. Age-Related Macular Degeneration: Epidemiology, Genetics, and Pathophysiology. Cell Prolif. 2022.
https://www.sciencedirect.com/science/article/pii/S2352304221000295 ScienceDirect - Zhang Z et al. (same as #6) – links oxidative stress, lipofuscin, NLRP3 inflammasome and complement activation in AMD. PubMed
4. Choriocapillaris Dropout & Vascular Dysregulation
- Lipecz A et al. From Mechanisms of Choriocapillaris Aging to Novel Interventions. GeroScience. 2019.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6925092/ PMC - Lutty GA. Choriocapillaris Dropout in Early Age-Related Macular Degeneration. Trans Vis Sci Tech. 2020.
https://pmc.ncbi.nlm.nih.gov/articles/PMC7216757/ PMC - Neri G et al. Choriocapillaris in Age-Related Macular Degeneration. Int J Mol Sci. 2024.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11590706/ PMC - Seddon JM et al. Choroidal Vascular Loss in Age-Related Macular Degeneration. JAMA Ophthalmol. 2016.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2553799 JAMA Network - Sohn EH et al. Choriocapillaris Degeneration in Geographic Atrophy. Am J Pathol. 2019.
https://pmc.ncbi.nlm.nih.gov/articles/PMC6616998/ ajp.amjpathol.org
5. VEGF-Driven Neovascularization & Anti-VEGF Therapy
- Cheng S et al. Treatment of Neovascular Age-Related Macular Degeneration: Anti-VEGF Agents and Emerging Therapies. Front Med. 2024.
https://www.frontiersin.org/articles/10.3389/fmed.2024.1411278/full Frontiers - Bidiwala S et al. Efficacy of Anti-VEGF Therapy in Neovascular AMD: A Review. 2024.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11684535/ PMC - Moraru AD et al. Guideline Recommendations for Optimal Anti-VEGF Therapy in Neovascular AMD. Life. 2024.
https://www.mdpi.com/2075-1729/14/10/1220 MDPI - Dervenis N et al. Neovascular Age-Related Macular Degeneration. BMJ Open Ophthalmol. 2024.
https://bmjophth.bmj.com/content/9/1/e001516 bmjophth.bmj.com
6. Drusen, Lipids & Barrier Formation
- Ban N et al. Drusen in AMD from the Perspective of Cholesterol Metabolism. Prog Retin Eye Res. 2024.
https://pmc.ncbi.nlm.nih.gov/articles/PMC11084323/ PMC - Apte RS et al. Targeting Tissue Lipids in Age-Related Macular Degeneration. Trends Mol Med. 2016.
https://www.sciencedirect.com/science/article/pii/S2352396416300342 ScienceDirect - Xu Q et al. Understanding AMD by Analogy: Systematic Review of Lipid-Related Extracellular Deposits. Lipids Health Dis. 2018.
https://lipidworld.biomedcentral.com/articles/10.1186/s12944-017-0647-7 SpringerLink - UMass Med – Punzo Lab. Age-Related Macular Degeneration (AMD).
https://www.umassmed.edu/punzolab/research/amd/ UMass Chan Medical School
7. Macular Pigment, Lutein/Zeaxanthin & Blue-Light Protection
- Lima VC et al. Macular Pigment in Retinal Health and Disease. Int J Retina Vitreous. 2016.
https://journalretinavitreous.biomedcentral.com/articles/10.1186/s40942-016-0044-9 SpringerLink - Whitehead AJ et al. Macular Pigment: A Review of Current Knowledge. Arch Ophthalmol. 2006.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/417803 JAMA Network - Scripsema NK et al. Lutein, Zeaxanthin, and Meso-Zeaxanthin in Clinical Practice. J Ophthalmol. 2015.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4706936/ PMC - Mrowicka M et al. Lutein and Zeaxanthin and Their Roles in AMD. Nutrients. 2022.
https://pmc.ncbi.nlm.nih.gov/articles/PMC8874683/ PMC - National Eye Institute (NEI). AREDS/AREDS2 Clinical Trials.
https://www.nei.nih.gov/research/clinical-trials/age-related-eye-disease-studies-aredsareds2/about-areds-and-areds2 National Eye Institute - Keenan TDL et al. Oral Antioxidant and Lutein/Zeaxanthin Supplements Slow GA Progression in AMD.Ophthalmology. 2025.
https://pubmed.ncbi.nlm.nih.gov/39025435/ PubMed - Evans JR, Cochrane Review. Antioxidant Vitamin and Mineral Supplements for Slowing AMD. 2023.
https://www.cochranelibrary.com/cdsr/doi/10.1002/14651858.CD000254.pub5/abstract Cochrane Library
8. Photoreceptor Degeneration & Functional Loss
- Curcio CA et al. Photoreceptor Loss in Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 1996.
https://pubmed.ncbi.nlm.nih.gov/8641827/ PubMed - Curcio CA et al. Photoreceptor Topography in Ageing and Age-Related Maculopathy. Eye. 2001.
https://www.nature.com/articles/eye2001140.pdf Nature - Jackson GR et al. Photoreceptor Degeneration and Dysfunction in Aging and ARM. Am J Ophthalmol. 2002.
https://pubmed.ncbi.nlm.nih.gov/12067593/ PubMed - Shelley EJ et al. Cone Degeneration in Aging and AMD. Arch Ophthalmol. 2009.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/422827 JAMA Network - Cheng SY et al. Altered Photoreceptor Metabolism Causes Late-Stage AMD-Like Pathology. PNAS. 2020.
https://www.pnas.org/doi/10.1073/pnas.2000339117 PNAS
9. Genetic Susceptibility (CFH, ARMS2, Complement Genes)
- Schwartz SG et al. Genetics and AMD: A Practical Review for Clinicians. Br J Ophthalmol. 2016.
https://pmc.ncbi.nlm.nih.gov/articles/PMC4938141/ PMC - Klein R et al. Risk Alleles in CFH and ARMS2 and Long-Term Incidence of AMD. Arch Ophthalmol. 2013.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/1390428 JAMA Network - Scholl HPN et al. CFH, C3 and ARMS2 Are Significant Risk Loci for Geographic Atrophy. PLoS ONE. 2009.
https://journals.plos.org/plosone/article?id=10.1371/journal.pone.0007418 PLOS - Kumaramanickavel G et al. Age-Related Macular Degeneration: Genetics and Biology. Semin Ophthalmol. 2016.
https://www.sciencedirect.com/science/article/pii/S2162098923003080 ScienceDirect
10. Systemic & Lifestyle Risk Factors (Smoking, BP, Metabolic Health)
- Babaker R et al. Risk Factors for Age-Related Macular Degeneration: Updated Systematic Review and Meta-Analysis. Medicine (Baltimore). 2025.
https://journals.lww.com/md-journal/fulltext/2025/02210/risk_factors_for_age_related_macular_degeneration_.71.aspx Lippincott Journals - Kuan V et al. Association of Smoking, Alcohol, Blood Pressure, BMI and Glycemic Traits with AMD. JAMA Ophthalmol. 2021.
https://jamanetwork.com/journals/jamaophthalmology/fullarticle/2785704 JAMA Network - Heesterbeek TJ et al. Risk Factors for Progression of Age-Related Macular Degeneration. Ophthalmic Physiol Opt. 2020.
https://onlinelibrary.wiley.com/doi/10.1111/opo.12675 Wiley Online Library - Saigal K et al. Modifiable Lifestyle Risk Factors and Strategies for Slowing AMD. Vision. 2025.
https://www.mdpi.com/2411-5150/9/1/16 MDPI - Macular Society. Nutrition and AMD.
https://www.macularsociety.org/support/daily-life/practical-guides/healthy-living/nutrition/ Macular Society


Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.