The Intertwined Relationship Between Age-Related Macular Degeneration and Cardiovascular Health
I. Introduction
Age-related macular degeneration (AMD) stands as the most prevalent cause of severe eyesight loss among individuals aged 50 and older, specifically impacting central vision and the ability to discern fine details . This condition is recognized as the leading cause of visual disability in developed nations and is projected to affect a staggering 288 million people globally by 2040 . While AMD rarely culminates in complete blindness, its profound impact on central vision significantly impairs daily activities essential for independence, such as reading, driving, and recognizing faces .
Concurrently, cardiovascular disease (CVD) represents a broad and encompassing term for a range of conditions that affect the heart or blood vessels . These conditions are frequently associated with the accumulation of fatty deposits, known as plaque, inside the arteries (atherosclerosis), which can narrow vessels and increase the risk of blood clots . CVD remains the single leading cause of death for both men and women worldwide and within the United States, underscoring its immense global health burden .
The escalating prevalence of both AMD and CVD, particularly within the aging demographic, points to a significant and increasing likelihood of these two conditions co-occurring within the same individual . Projections indicate a substantial rise in the number of affected individuals by 2040-2050 . This demographic trend is not merely a statistical overlap but represents a growing public health challenge characterized by multimorbidity, where the simultaneous presence of multiple chronic conditions complicates diagnosis, treatment, and overall patient outcomes . The fact that AMD is the leading cause of visual disability and CVD is the leading cause of mortality globally means that their co-occurrence has profound implications for both the quality of life and the lifespan of affected individuals. This pervasive co-occurrence necessitates a fundamental shift in healthcare paradigms. Instead of managing these conditions in isolation, a more integrated, holistic approach to care for aging adults is imperative. Recognizing that ocular health can serve as a "window" into systemic vascular health can facilitate earlier detection and more effective, coordinated management strategies.
This article aims to provide a comprehensive and evidence-based exploration of the complex and increasingly recognized relationship between Age-Related Macular Degeneration and Cardiovascular Disease. It will delve into their shared risk factors, elucidate the underlying pathophysiological mechanisms that link them, review the epidemiological evidence of their association, discuss crucial clinical implications for patient management, and outline promising future directions for research and integrated care strategies.
II. Understanding Age-Related Macular Degeneration (AMD)
Age-related macular degeneration (AMD) is characterized by damage to the macula, a critical part of the retina responsible for sharp, central vision . This damage impairs the ability to see fine details, whether objects are near or far . AMD is the most common cause of severe vision loss among individuals aged 50 and older . Its prevalence is substantial, affecting approximately 11 million individuals in the United States alone, with a global prevalence estimated at 170 million. Projections indicate a significant increase, with 22 million affected in the U.S. by 2050 and a global prevalence reaching 288 million by 2040 .
Types of AMD: Dry (atrophic) and Wet (neovascular) AMD
AMD manifests in two primary forms, each with distinct characteristics and progression patterns:
-
Dry AMD: This form is the most common, accounting for approximately 80% of all AMD cases . Dry AMD progresses as the light-sensitive cells in the macula slowly break down, typically affecting one eye at a time, leading to a slow and gradual loss of central vision . A hallmark of dry AMD is the presence of tiny yellow protein deposits called drusen and thinning of the macula . It is believed that age-related damage to an important support membrane beneath the retina contributes to its development . Recent advancements in treatment for late-stage atrophic AMD, specifically geographic atrophy (GA), include the FDA approval of complement inhibitors like pegcetacoplan (February 2023) and avacincaptad pegol (August 2023). These drugs target the complement cascade, a part of the immune system implicated in AMD pathogenesis, and have shown promise in slowing the progression of GA by approximately 20%, although they do not restore lost vision .
-
Wet AMD: Although less common, wet AMD is generally more severe and leads to more rapid and significant vision loss than the dry form . This type is characterized by the growth of abnormal, fragile blood vessels beneath the retina. These vessels are prone to leaking fluid and blood, which can cause scarring of the macula and result in a large blind spot in the center of the visual field . Current therapeutic strategies for wet AMD primarily focus on addressing choroidal neovascularization (CNV), often through anti-vascular endothelial growth factor (anti-VEGF) injections .
Key Characteristics, Symptoms, and Diagnostic Methods
The presentation of AMD can vary, but certain symptoms and diagnostic findings are common:
-
Symptoms: Common symptoms of AMD include blurry or fuzzy vision, difficulty recognizing familiar faces, and a distinctive distortion where straight lines appear wavy . Patients may also experience a dark, empty area or a blind spot in the center of their vision . The loss of central vision is particularly debilitating as it is essential for activities requiring fine detail perception, such as driving, reading, and performing close-up work . Notably, peripheral (side) vision remains unaffected, preserving the ability to navigate without bumping into objects .
-
Early Signs: One of the most common early signs of AMD is the presence of drusen, tiny yellow deposits observed in the retina . Pigmentary abnormalities in the retinal pigment epithelium (RPE) are also early indicators .
-
Diagnosis: Diagnosis typically involves a comprehensive eye exam and medical history . Key diagnostic tests include:
- Visual Acuity Test: A standard eye chart test to measure vision at various distances .
- Pupil Dilation: Eyedrops are used to widen the pupil, allowing for a detailed examination of the retina .
- Fluorescein Angiography: Primarily used for wet AMD, this test involves injecting a special dye into an arm vein. Pictures are then taken as the dye passes through retinal blood vessels, helping to identify leaking vessels that may require treatment .
- Amsler Grid Test: This grid helps patients self-monitor for changes in central vision, such as blurry, distorted, or blank spots, and is a crucial tool for early detection of vision changes .
The description of wet AMD clearly highlights abnormal blood vessel growth and leakage in the macula . Furthermore, the characteristic drusen deposits in dry AMD have been noted to resemble atherosclerotic plaques in their composition. This suggests that the retina, particularly the macula, is not merely a passive recipient of age-related degeneration but an active site where systemic vascular processes can manifest. The retinal pigment epithelium (RPE), which is crucial for retinal health, exhibits high metabolic demand and is particularly prone to oxidative stress , making it vulnerable to systemic microvascular insults. This perspective is crucial because it shifts AMD from being perceived solely as an "eye disease" to a potential localized manifestation of broader systemic microvascular dysfunction. This conceptualization parallels how kidney disease or peripheral neuropathy can be manifestations of systemic conditions like hypertension or diabetes. This reinforces the idea of shared pathophysiology with cardiovascular disease and suggests that the eye can serve as an accessible "window" to systemic vascular health.
Early stages of AMD often present with no noticeable symptoms , emphasizing the critical importance of regular visits to an ophthalmologist for screening . The recent FDA approvals of treatments for geographic atrophy underscore that early intervention can effectively slow disease progression, preserving vision. If AMD is indeed an ocular indicator of systemic vascular issues, then the early detection of AMD could serve as a trigger for earlier and more aggressive screening and management of cardiovascular disease risk factors. This suggests a proactive and expanded role for ophthalmologists in identifying patients who may be at higher systemic risk for CVD. Early AMD detection could lead to timely referrals to cardiologists or primary care physicians for comprehensive cardiovascular evaluations, potentially benefiting both ocular and cardiovascular health outcomes. This fosters a more integrated and preventative approach to patient care.
III. Understanding Cardiovascular Disease (CVD)
Cardiovascular disease (CVD) is a broad umbrella term encompassing a range of conditions that affect the heart and blood vessels throughout the body . The primary underlying cause for many forms of CVD is atherosclerosis, a condition where fatty deposits, cholesterol, and other substances accumulate to form plaque within the walls of arteries . Over time, this plaque buildup can narrow and harden the arteries, limiting or blocking blood flow and leading to a variety of serious health problems .
Common Forms of CVD
CVD manifests in several common forms, each with distinct impacts on the body:
-
Coronary Heart Disease (CHD) / Coronary Artery Disease (CAD): This is the most common type of heart disease, occurring when plaque builds up in the coronary arteries that supply blood to the heart muscle . This narrowing restricts blood flow and oxygen to the heart, which can manifest as angina (chest pain) . Untreated, CAD can lead to severe complications such as heart attacks (where blood flow is suddenly blocked), heart failure (where the heart cannot pump blood effectively), or arrhythmias (abnormal heart rhythms) .
-
Stroke: A stroke occurs when the blood supply to a part of the brain is interrupted or cut off, leading to brain damage . A transient ischaemic attack (TIA), or "mini-stroke," is similar but involves only a temporary disruption of blood flow . Stroke shares many of the same risk factors as other forms of heart disease .
-
Heart Failure (HF): This condition develops when the heart muscle becomes stiff or weak, impairing its ability to pump enough oxygen-rich blood to meet the body's needs . Symptoms can affect the entire body. High blood pressure and coronary artery disease are common causes of heart failure .
-
Peripheral Arterial Disease (PAD): PAD occurs when plaque buildup narrows the arteries supplying blood to the limbs, most commonly the legs and feet . This reduced blood flow can cause symptoms such as dull or cramping leg pain (especially with walking), hair loss on the legs and feet, numbness, weakness, and persistent ulcers .
-
Hypertension (High Blood Pressure): Hypertension is a cardiovascular disease in itself, characterized by excessively high pressure of blood within the arteries and other blood vessels . If uncontrolled, high blood pressure significantly damages blood vessels throughout the body and is a major risk factor for developing other severe CVDs, including heart attack, heart failure, stroke, and kidney disease .
Other forms of CVD include arrhythmias (problems with heart rate or rhythm), aortic diseases (conditions affecting the aorta, the body's largest blood vessel, such as aneurysms), and heart valve diseases (where one or more heart valves do not function properly) .
Major Risk Factors for CVD
A multitude of factors contribute to the development of CVD, categorized as modifiable and non-modifiable:
-
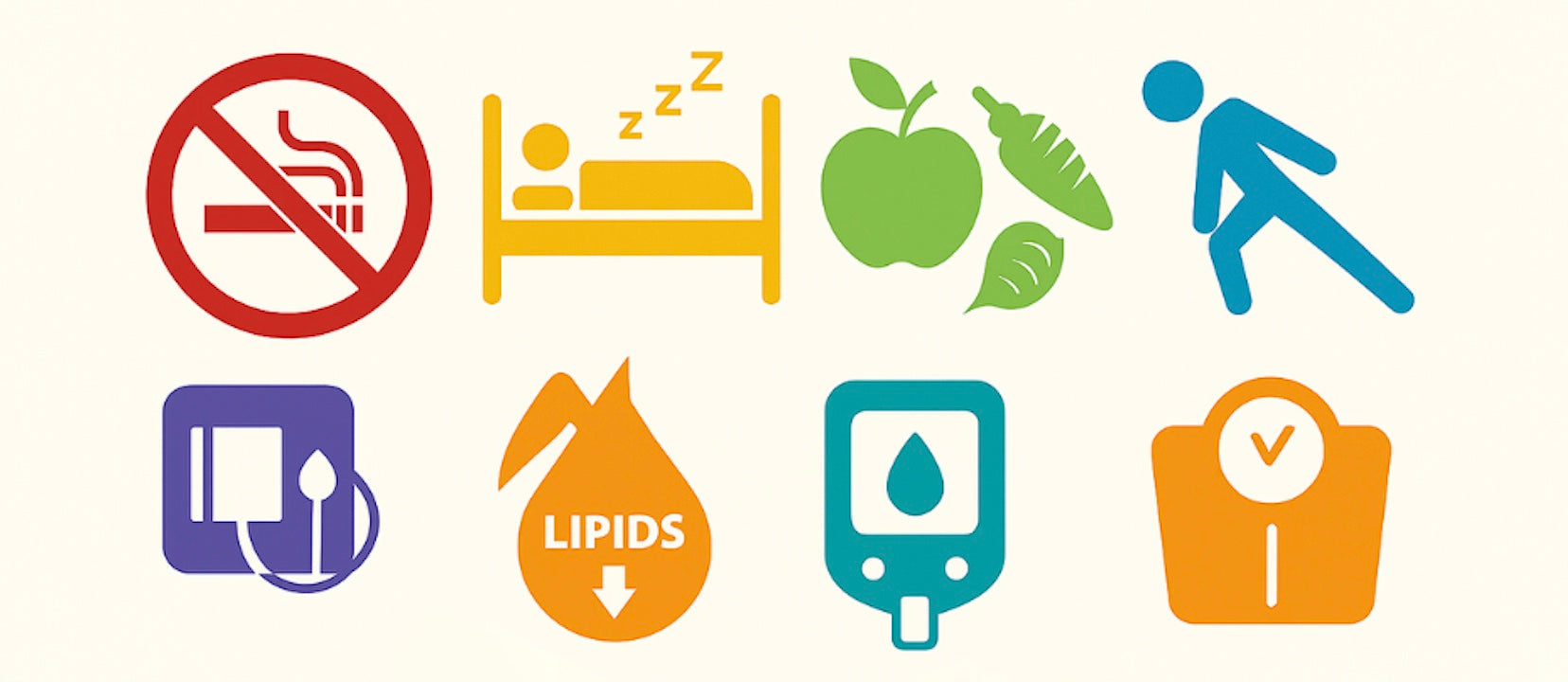
Modifiable Risk Factors: These include high blood pressure, unhealthy blood cholesterol levels, smoking and tobacco use, diabetes mellitus, obesity (excess body fat), an unhealthy diet (particularly high in saturated fats, trans fats, cholesterol, and sodium), insufficient physical activity, and excessive alcohol consumption . These factors can often be managed or altered through lifestyle changes and medical interventions.
-
Non-Modifiable Risk Factors: These are factors that cannot be changed. They include advancing age (risk increases with age) , genetics and family history (genetic factors likely play a role, often combined with shared environmental factors) , and certain racial and ethnic groups (e.g., African Americans, American Indians and Alaska Natives, White people, and some subgroups of the Asian American population experience higher rates of adverse cardiovascular risk factors and outcomes) .
The consistent emphasis on atherosclerosis as the fundamental underlying pathology for diverse CVD manifestations, including coronary heart disease, stroke, and peripheral artery disease , highlights that CVD is not a collection of isolated organ-specific problems. Instead, it is a pervasive systemic vascular disorder. This systemic nature implies that the same pathological processes affecting large and medium-sized arteries throughout the body could also impact smaller vascular beds, such as those supplying the retina. This understanding reinforces the biological plausibility of a strong connection between CVD and AMD. It suggests that interventions aimed at preventing or reversing systemic atherosclerosis, such as lipid-lowering therapies or blood pressure control, could have widespread benefits, potentially extending to the microvasculature of the eye and influencing AMD progression.
The evidence clearly indicates that a significant proportion of CVD-related morbidity and mortality is attributable to modifiable risk factors . Despite this, projections show an alarming increase in the prevalence of key CVD risk factors like hypertension, diabetes, and obesity by 2050 . This widening gap between preventable causes and rising prevalence signals a critical and worsening public health crisis. This underscores the urgent need for robust public health campaigns and clinical interventions focused on widespread adoption of healthy lifestyle behaviors and aggressive management of established risk factors. If these modifiable risk factors are indeed shared with AMD, as suggested, then a unified public health strategy could offer a powerful "dual prevention" approach, mitigating the burden of both cardiovascular disease and age-related vision loss.
IV. Shared Risk Factors and Commonalities
The strong epidemiological and biological links between Age-Related Macular Degeneration (AMD) and Cardiovascular Disease (CVD) are underscored by a significant overlap in their respective risk factor profiles. This convergence suggests that both conditions may arise from common underlying systemic vulnerabilities, a concept often referred to as the "common soil" hypothesis.
Detailed Discussion of Overlapping Modifiable Risk Factors
-
Age: Advancing age is the single most significant and universal non-modifiable risk factor for both AMD and CVD . The risk for both conditions increases substantially after 50 years of age, and their prevalence is projected to rise dramatically with the global aging population .
-
Smoking: Cigarette smoking and other forms of tobacco use are well-established, potent risk factors for both AMD and CVD . The harmful substances in tobacco directly damage and narrow blood vessels throughout the body, including those supplying the eye, and nicotine specifically elevates blood pressure, contributing to systemic vascular dysfunction .
-
Hypertension (High Blood Pressure): High blood pressure is a major, modifiable risk factor for CVD, leading to damage to blood vessels and increasing the risk of heart attack, heart failure, and stroke . Crucially, hypertension has also been consistently linked to an increased risk of developing AMD. This connection likely stems from the detrimental effects of high pressure on the delicate ocular vasculature.
-
High Cholesterol / Dyslipidemia: Elevated levels of cholesterol and other lipids in the blood (dyslipidemia) contribute to the narrowing of blood vessels and increased risk of blood clot formation, central to CVD pathogenesis . In AMD, high cholesterol levels have been identified as a risk factor , and the lipid-rich composition of drusen deposits, a hallmark of AMD, further suggests a direct role for lipid metabolism in its development.
-
Obesity / Unhealthy Diet: A diet high in saturated fat is explicitly listed as a risk factor for AMD . Obesity, defined as excess body fat, is strongly associated with adverse lipid profiles (higher "bad" cholesterol and triglycerides, lower "good" cholesterol), high blood pressure, and diabetes, all of which are major CVD risk factors . High Body Mass Index (BMI) has also been linked to an increased risk for late AMD.
-
Diabetes Mellitus: Diabetes, a chronic condition characterized by high blood sugar levels, damages blood vessels throughout the body, making them more prone to narrowing and increasing the risk for various CVDs . Diabetes is also identified as a risk factor for AMD .
-
Physical Inactivity: A sedentary lifestyle is a significant contributor to several modifiable CVD risk factors, including obesity, high blood pressure, high cholesterol, and diabetes . Conversely, regular physical exercise has been shown to have protective associations against the incidence and progression of AMD.
Non-Modifiable Shared Risk Factors
-
Genetics and Family History: A family history of AMD significantly increases an individual's risk of developing the condition . Similarly, genetic factors play a demonstrable role in predisposing individuals to high blood pressure, heart disease, and other related cardiovascular conditions . Common genetic variants, such as those involving apolipoprotein E and complement factor H, have been implicated in the pathogenesis of both CVD and AMD, highlighting shared genetic predispositions, though their exact mechanisms and interactions are complex and not always identical.
-
Race and Ethnicity: While AMD is more prevalent in White populations , heart disease remains the leading cause of death for most racial and ethnic groups in the United States, including African Americans, American Indians and Alaska Natives, and White people . Certain racial and ethnic groups also experience markedly higher rates of adverse cardiovascular risk factors and worse outcomes, indicating a complex interplay of genetic, environmental, and socioeconomic factors .
The overwhelming evidence of shared modifiable and non-modifiable risk factors between AMD and CVD strongly suggests that these two seemingly disparate diseases are not merely co-occurring by chance. Instead, they likely stem from a "common soil" of systemic physiological vulnerabilities. This implies that the same underlying biological stressors, environmental exposures, and genetic predispositions affect multiple organ systems concurrently, including the delicate structures of the eye and the intricate cardiovascular network. This profound understanding provides a robust rationale for integrated prevention and management strategies. Interventions that target these shared risk factors – such as smoking cessation, adoption of a heart-healthy diet, regular physical activity, and pharmacological control of hypertension and dyslipidemia – are highly likely to yield dual benefits, simultaneously improving both ocular health and cardiovascular outcomes. This unified approach can simplify patient education and empower individuals with a more cohesive and effective health management strategy.
Age is consistently highlighted as the most significant and pervasive risk factor for both AMD and CVD . This suggests that the fundamental processes of aging itself, with its associated cellular senescence, molecular damage accumulation, and immunosenescence (age-related changes in the immune system), serve as a profound underlying driver for the development and progression of both conditions. The observation that AMD is associated with an increased risk of all-cause mortality further supports the idea that AMD may not be an isolated ocular pathology but rather a visible marker or manifestation of broader systemic biological aging processes and overall frailty. This underscores the importance of research into the fundamental biology of aging as a potential avenue for developing novel interventions that could prevent or significantly delay the onset and progression of both AMD and CVD. It also reinforces the critical importance of adopting healthy lifestyle habits throughout life, as cumulative damage and chronic low-grade inflammation associated with unhealthy aging contribute significantly to the development of these age-related degenerative diseases.
Table 1: Shared Risk Factors for Age-Related Macular Degeneration and Cardiovascular Disease
V. Pathophysiological Mechanisms Linking AMD and CVD
Beyond shared risk factors, a growing body of evidence points to common underlying pathophysiological mechanisms that contribute to the development and progression of both Age-Related Macular Degeneration (AMD) and Cardiovascular Disease (CVD). These shared biological pathways suggest that AMD may not be an isolated ocular disease but rather a manifestation of systemic processes that also impact cardiovascular health.
Chronic Inflammation
Chronic inflammation is increasingly recognized as a central and plausible biological mechanism underlying the pathogenesis of both AMD and CVD. Systemic markers of inflammation, such as C-reactive protein (CRP), are well-established independent risk factors for CVD and are also significantly associated with AMD. High levels of CRP have been shown to predict adverse cardiovascular events, including death from myocardial infarction . In the context of AMD, the activation of the complement system, a crucial part of the innate immune response, plays a significant role in the formation of drusen, the characteristic deposits in the macula. Genetic variants of complement proteins are strong risk factors for AMD. Similarly, dysregulated immune responses and chronic low-grade inflammation are fundamental contributors to the development and progression of atherosclerosis, the primary driver of many CVDs . Inflammatory processes are intricately linked to endothelial (vascular) dysfunction, which is an early and critical step in the development of various CVDs, including hypertension, hypercholesterolemia, and coronary artery disease. This suggests a systemic inflammatory milieu that impacts both the retinal and cardiovascular systems.
Oxidative Stress
Increased generation of reactive oxygen species (ROS) and an imbalance between ROS production and antioxidant defense mechanisms (oxidative stress) are crucial contributors to the proatherogenic processes of vascular dysfunction and atherothrombosis in CVD. In the context of AMD, the retinal pigment epithelium (RPE) is uniquely susceptible to oxidative stress. This vulnerability stems from its high oxygen exposure, intense metabolic demand, and the continuous generation of ROS during the phagocytosis of photoreceptor outer segments . Mitochondrial oxidative stress within the RPE is known to contribute to metabolic dysfunction in both the RPE and photoreceptors, leading to cellular damage . Most cardiovascular diseases are characterized by this imbalance in redox homeostasis. Oxidative stress directly leads to endothelial dysfunction, which is considered an early and significant predictor of cardiovascular events. Furthermore, there is a tight and complex association between redox regulatory pathways and inflammation, where mitochondrial superoxide can trigger the activation of immune cells and subsequent inflammatory cascades, creating a reinforcing feedback loop.
The consistent highlighting of inflammation and oxidative stress not as isolated factors, but as deeply intertwined and mutually reinforcing processes in both AMD and CVD indicates a deeper causal relationship beyond mere correlation. Oxidative stress can initiate and exacerbate inflammatory responses, while chronic inflammation can, in turn, increase oxidative stress, creating a detrimental feedback loop. This "redox crosstalk" is a critical underlying mechanism. The concept of "immunosenescence," where plasma concentrations of inflammatory markers increase with age independently of other risk factors, provides a direct mechanistic link to the universal risk factor of aging. This profound understanding suggests that therapeutic strategies targeting this vicious cycle – for example, through potent anti-inflammatory agents or novel antioxidant therapies – could offer highly effective interventions for preventing or treating both AMD and CVD. This moves beyond single-pathway interventions and emphasizes the potential for broad-spectrum benefits from targeting fundamental systemic dysregulations.
Vascular Dysfunction / Atherosclerosis
Endothelial (vascular) dysfunction, characterized by impaired function of the inner lining of blood vessels, is an early correlate for coronary artery disease and is prevalent in many low-grade inflammatory diseases (e.g., rheumatoid arthritis, type 2 diabetes), accelerating atherosclerosis and increasing cardiovascular mortality. Atherosclerosis itself is hypothesized to play a direct pathogenic role in AMD development due to its impact on choroidal circulation and the deposition of lipids at Bruch's membrane, a critical layer beneath the retina. The characteristic drusen deposits in AMD exhibit remarkable commonalities in their composition with systemic atherosclerotic plaques. Both contain components such as lipids, proteins (e.g., apolipoproteins, complement components), and calcium. This striking resemblance strongly suggests shared pathophysiologies, although the primary source of lipids in drusen is local (from RPE and photoreceptors), while in atherosclerotic plaques, it is systemic (from circulation). The "hemodynamic model" of AMD proposes that lipid deposition within the sclera (the white outer layer of the eye) can increase scleral stiffness and choroidal vascular resistance. This leads to decreased blood flow in the choroid (the vascular layer supplying the retina), which can induce choroidal neovascularization (CNV), a key feature of wet AMD. This model directly links systemic vascular health to ocular pathology.
The striking resemblance between drusen deposits in AMD and systemic atherosclerotic plaques, both in terms of their lipid-rich composition and the involvement of inflammatory and complement components, is a powerful conceptual link. When combined with the recognized role of lipid deposition and vascular dysfunction in both conditions, it strongly suggests that AMD can be conceptualized as a localized form of atherosclerosis specifically affecting the microvasculature of the eye. The "hemodynamic model" further supports this vascular perspective by linking choroidal blood flow changes to AMD progression. This conceptualization has significant implications for both research and clinical practice. It could lead to the development of novel therapeutic approaches for AMD that draw lessons and insights from established atherosclerosis management strategies. For instance, interventions aimed at reducing systemic lipid burden, improving endothelial function, or modulating systemic inflammation might have direct and beneficial effects on retinal health. Furthermore, it strengthens the argument for ophthalmologists to consider a patient's systemic vascular health comprehensively when diagnosing and managing AMD.
Genetic Predisposition
While complex, common genetic variants have been identified that are linked to both CVD and AMD. Notable examples include variants in apolipoprotein E and complement factor H. However, the precise mechanisms by which these genetic factors influence both conditions are not always identical, and their roles can be complex. For instance, while earlier studies suggested a direct link between AMD-associated variants in complement factor H and coronary heart disease, a more recent meta-analysis did not consistently confirm this direct association, highlighting the intricate nature of these genetic relationships.
VI. Epidemiological Evidence of Association
The epidemiological relationship between Age-Related Macular Degeneration (AMD) and Cardiovascular Disease (CVD) has been a subject of extensive research, yielding findings that, while sometimes conflicting, increasingly point towards a significant association, especially when considering specific disease stages and study methodologies.
Review of Cohort Studies and Meta-Analyses on the Association
Early epidemiological studies on the direct association between AMD and CVD, as well as associated mortality, have often presented conflicting results, contributing to ongoing debate.
-
All-cause Mortality: A meta-analysis encompassing 20 population-based cohort studies provided compelling evidence that AMD is associated with an approximately 8% increased risk of all-cause mortality . This finding suggests that AMD may serve as a marker reflecting broader systemic processes associated with biological aging and underlying serious somatic factors or diseases .
-
Overall CVD Incidence: A comprehensive meta-analysis of 13 cohort studies (comprising 8 prospective and 5 retrospective studies) found that early AMD was associated with a statistically significant 15% increased risk of total CVD. For late AMD, the initial pooled relative risk was 1.17. However, when the analysis was rigorously restricted to the subset of prospective studies, a markedly stronger association emerged, showing a 66% increased risk of CVD (Relative Risk, 1.66; 95% Confidence Interval [CI], 1.31–2.10). This suggests that retrospective studies, with their inherent methodological limitations, may obscure the true strength of this association.
-
Coronary Heart Disease (CHD): One meta-analysis indicated that early AMD was associated with a significant 19% increased risk of CHD. For late AMD, the association was not statistically significant in the primary analysis, but it was elevated when retrospective studies were excluded. Conversely, another meta-analysis found no significant relationship between AMD and incident CHD when data from both prospective and retrospective cohort studies were summarized .
-
Stroke: The pooled relative risk for stroke associated with AMD was 1.13 in one meta-analysis, although this was not statistically significant . Another meta-analysis reported that early AMD was associated with a pooled RR of 1.11 for stroke, and this risk became statistically significant for late AMD (RR, 1.43; 95% CI, 1.02–2.00) when retrospective studies were excluded. While some studies have found no association between AMD and stroke, others have reported significant links, including a notable 42% higher risk of cerebral infarction observed in the ARIC (Atherosclerosis Risk in Communities) study.
-
Heart Failure (HF): Recent studies have provided strong evidence for a significant association between AMD and heart failure. Both nonexudative (dry) and exudative (wet) forms of AMD were associated with a 1.58-fold increased risk of HF, even after adjusting for a wide range of potential confounders, including age, sex, socioeconomic status, hypertension, diabetes mellitus, hyperlipidemia, and coronary artery disease. This finding suggests that AMD acts as an independent predictor of HF risk.
Discussion of Conflicting Findings and Factors Influencing Study Outcomes
The observed inconsistency in epidemiological findings across various studies highlights the inherent complexity of the relationship between AMD and CVD. Several factors are believed to influence these study outcomes, including differences in study design (e.g., the inclusion of retrospective versus prospective cohorts), variations in participant age at baseline, the duration of follow-up, and crucially, the precision in defining and classifying AMD stages (e.g., early vs. late AMD).
A particularly important nuance is the role of visual disability. A nationwide cohort study revealed that while general AMD might not consistently show an increased risk of overall CVD, AMD when accompanied by visual disability was significantly associated with an increased risk of overall CVD (adjusted Hazard Ratio, 1.17; 95% CI, 1.06–1.29), myocardial infarction (aHR, 1.18), and ischemic stroke (aHR, 1.20). This trend was more pronounced in women and individuals with pre-existing cardiometabolic comorbidities . This critical distinction suggests that the severity of AMD, particularly as it impacts functional vision, may be a more potent indicator of systemic CVD risk than the mere presence of AMD itself.
The initial conflicting epidemiological results are a crucial point of discussion. The meta-analyses consistently demonstrate that when studies are methodologically more robust (e.g., prioritizing prospective designs over retrospective ones) or when the analysis is more granular (e.g., focusing on late-stage AMD or AMD with visual disability), the associations with CVD outcomes become significantly stronger and more consistent. This implies that earlier, less rigorous studies or those that aggregated all AMD stages together might have inadvertently diluted or obscured the true and clinically meaningful relationship between these two conditions. This highlights a critical need for future epidemiological research to prioritize prospective study designs, ensure sufficiently long follow-up periods, and implement precise phenotyping of AMD stages and severity, especially the presence and degree of visual disability. Such methodological rigor is indispensable for accurately delineating the complex AMD-CVD link, establishing potential causal pathways, and ultimately informing robust clinical guidelines and public health recommendations.
The finding that AMD with visual disability significantly increases the risk of overall CVD, myocardial infarction, and ischemic stroke, while general AMD might not, is a profound and actionable observation. Visual disability is not merely an ocular symptom; it can lead to a cascade of negative systemic effects, including reduced physical activity, increased frailty, higher rates of accidents and falls, and social isolation, all of which are independently linked to increased morbidity and all-cause mortality . This suggests a detrimental feedback loop where severe ocular disease actively contributes to overall systemic decline and increased vulnerability to cardiovascular events. This finding elevates visual disability from a mere symptom to a critical clinical marker for heightened systemic vulnerability. Patients presenting with advanced AMD and significant vision loss should be identified as a high-risk group for cardiovascular disease, necessitating aggressive screening, comprehensive cardiovascular evaluations, and proactive preventive measures. This underscores the broader, systemic impact of vision loss on an individual's overall health and quality of life, emphasizing the need for integrated care that addresses both ocular and systemic health challenges.
Table 2: Summary of Epidemiological Associations between AMD Stages and CVD Outcomes
VII. Clinical Implications and Integrated Management Strategies
The compelling evidence for shared risk factors, common pathophysiological mechanisms, and epidemiological associations between Age-Related Macular Degeneration (AMD) and Cardiovascular Disease (CVD) necessitates a fundamental shift in clinical practice. An integrated approach, emphasizing cross-disciplinary awareness and comprehensive patient management, is crucial for optimizing outcomes in an aging population grappling with multimorbidity.
Importance of Cross-Disciplinary Awareness Among Healthcare Professionals
Given the significant overlap in risk factors and underlying pathophysiological mechanisms, a holistic approach to patient care is paramount . It is increasingly important for clinicians across specialties, particularly ophthalmologists and cardiologists, to be acutely aware of the intricate interconnections between AMD and CVD . This awareness fosters a more comprehensive understanding of a patient's overall health status, moving beyond organ-specific silos.
Recommendations for Early Detection of AMD and Its Potential Role as an Indicator for CVD Risk
Early detection of AMD is inherently beneficial due to the availability of treatments that can slow disease progression, particularly for geographic atrophy. Beyond ocular benefits, the evidence suggests that screening for AMD could serve as a potentially valuable strategy for the early identification of individuals at higher risk for cardiovascular conditions, especially heart failure. AMD, even independent of other cardiometabolic diseases, has been shown to be associated with an increased risk of heart failure. Crucially, patients diagnosed with AMD, particularly those experiencing visual disability, should be proactively targeted for comprehensive CVD prevention and aggressive risk factor management.
The retina offers a unique and non-invasive opportunity to observe the microvasculature, providing insights into systemic microvascular disease . The strong and independent association between AMD (especially when accompanied by visual disability) and an increased risk of heart failure and other CVD events suggests that the presence of AMD, particularly advanced stages, can serve as an early warning signal for broader cardiovascular issues that might otherwise go undetected. This elevates the ophthalmologist's role beyond just ocular health. They can become crucial partners in identifying patients who are at higher systemic cardiovascular risk, prompting timely referrals to cardiologists or primary care physicians for comprehensive cardiovascular evaluation and aggressive management. This fosters a truly integrated healthcare model, where a diagnosis in one specialty triggers proactive screening and intervention in another, ultimately optimizing patient outcomes and reducing the overall burden of chronic disease.
Emphasis on Comprehensive Risk Factor Management
The extensive overlap in modifiable risk factors for both AMD and CVD presents a significant opportunity. If managing these risk factors effectively improves cardiovascular outcomes , it logically follows that these same lifestyle and pharmacological interventions could concurrently exert beneficial effects on AMD progression. The direct evidence that enhanced cardiovascular health (CVH) metrics are significantly associated with a reduced risk of developing AMD provides compelling support for this "dual prevention" concept. This provides a strong clinical imperative for aggressive, holistic risk factor modification. Instead of treating AMD and CVD as separate, unrelated entities, clinicians should increasingly view them as interconnected manifestations of systemic health. This perspective enables a unified health strategy where a single, comprehensive intervention plan can have beneficial ripple effects across multiple organ systems, simplifying patient education and potentially improving adherence to treatment.
Key recommendations include:
-
Lifestyle Modifications: These form the cornerstone of management for both conditions. Key recommendations include:
- Smoking Cessation: Complete avoidance of smoking and all tobacco products is critical due to their damaging effects on blood vessels .
- Heart-Healthy Diet: Adopting a diet low in sodium, saturated fat, trans fat, and sugar, such as the Mediterranean diet, is proven to lower the risk of heart attack and stroke and is beneficial for AMD .
- Regular Physical Activity: Aiming for at least 30 minutes of moderate-intensity aerobic exercise five days a week can significantly lower the risk of heart disease and has protective associations against AMD progression.
- Weight Management: Maintaining a healthy weight is crucial, as obesity is linked to multiple CVD risk factors and increased risk for late AMD.
- Alcohol Moderation: Limiting alcohol consumption can help manage blood pressure and triglyceride levels .
-
Pharmacological Interventions: Appropriate medication management is essential. This includes prescribing drugs to lower blood pressure, reduce cholesterol levels (e.g., statins, which have also shown variable associations with AMD progression), and effectively manage diabetes. Optimal management of these cardiometabolic comorbidities is imperative in high-risk patient populations .
-
Holistic Cardiovascular Health (CVH): Adherence to comprehensive cardiovascular health metrics, such as the American Heart Association's Life's Essential 8 (LE8) score, has been significantly associated with a reduced risk of developing AMD . Achieving optimal CVH across these metrics could potentially avert a notable percentage of AMD cases, highlighting the broad benefits of a unified approach to health .
The Role of a Multidisciplinary Approach in Patient Care
The increasing prevalence of multimorbidity, where patients present with two or more chronic conditions, complicates diagnosis and treatment, particularly in older adults . To address this complexity, multidisciplinary teams are increasingly recognized as effective and are advised for managing complex conditions like CVD . These teams typically comprise a range of healthcare professionals, including cardiologists, specialized cardiovascular nurses, general practitioners, pharmacists, dieticians, exercise physiologists, and psychologists . This integrated team approach ensures collaborative evaluation of treatment options, development of individualized patient-specific treatment plans, and effective patient education. Such collaborative care has been shown to improve patient outcomes, alleviate suffering, and enhance the overall experience of care for both patients and their families .
VIII. Recent Advances and Future Directions
The evolving understanding of the intertwined nature of Age-Related Macular Degeneration (AMD) and Cardiovascular Disease (CVD) is driving significant advancements in both research and clinical practice. Future efforts are focused on leveraging shared pathophysiological insights to develop more effective, integrated management strategies.
Overview of Novel Therapeutic Targets for AMD
For neovascular (wet) AMD, anti-vascular endothelial growth factor (anti-VEGF) therapies have revolutionized treatment, significantly preserving vision for millions of patients . A major recent breakthrough for geographic atrophy (GA), a severe form of dry AMD, occurred with the FDA approval of novel complement inhibitors: pegcetacoplan (SYFOVRE®, February 2023) and avacincaptad pegol (IZERVAY®, August 2023) . These drugs specifically target the complement cascade, a component of the immune system identified as a primary cause of AMD, and have demonstrated the ability to slow the development of GA by approximately 20% . While they do not restore lost vision, they represent a crucial step in addressing a previously untreatable form of advanced dry AMD.
Ongoing Research into Shared Pathophysiological Pathways and Common Therapeutic Targets
Current research efforts are intensely focused on gaining a more comprehensive understanding of the primary pathomechanisms underlying AMD onset and progression. The ultimate goal is to identify innovative and effective therapeutic targets that can significantly improve patient outcomes . The intricate and mutually reinforcing link between inflammation and oxidative stress, identified as core drivers in both AMD and CVD, presents promising avenues for common therapeutic targets. Ongoing clinical trials are actively investigating anti-inflammatory strategies for CVD. For instance, drugs like canakinumab and colchicine have demonstrated efficacy in secondary cardiovascular prevention by modulating specific inflammatory pathways, such as the NLRP3 inflammasome/IL-1β axis. These anti-inflammatory approaches are now being explored for their potential application across a broader spectrum of CVDs, offering insights that could be transferable to AMD. Further elucidation of shared mechanisms and specific metabolic pathways, particularly the lipid-cholesterol pathway, is expected to inform the development of early risk stratification algorithms and identify novel targets for prevention and intervention that benefit both AMD and CVD. Research into the "heart-brain axis," specifically in the context of Alzheimer's Disease and CVD, also highlights shared risk factors (e.g., hypertension, inflammation, dyslipidemia) and the potential for common prevention strategies that could extend to AMD .
The recent FDA approvals of complement inhibitors for geographic atrophy in AMD and the ongoing success of clinical trials targeting inflammatory pathways in CVD represent significant breakthroughs in their respective fields. Given the identified shared fundamental pathophysiological pathways, particularly chronic inflammation and oxidative stress, these advancements suggest a powerful potential for therapeutic transfer or synergy. Treatments proven effective in one condition, especially those targeting core mechanisms like the complement system or specific inflammatory cascades (e.g., NLRP3 inflammasome/IL-1β ), could be repurposed or inspire novel therapies for the other. The concept of "redox hubs" in inflammation further points to specific molecular targets that could yield dual benefits. This highlights the immense value and necessity of intensified collaborative research efforts between ophthalmology and cardiology. Rapid translation of findings, particularly concerning novel mechanistic targets, from one field to the other could significantly accelerate drug discovery, optimize existing treatments, and ultimately lead to improved patient outcomes for both AMD and CVD.
The Potential for Integrated Care Models and Precision Medicine Approaches
The increasing prevalence of multimorbidity, where patients present with multiple chronic conditions, necessitates the development of better clinical guidance for managing these complex cases . Integrated care models, such as multidisciplinary "heart teams" already implemented for complex CVD patients, represent a successful paradigm . These models could be expanded to formally include ocular health specialists, fostering seamless collaboration for patients with co-occurring AMD and CVD. Precision medicine, which aims to tailor medical treatment to the individual characteristics of each patient, holds immense promise . A deeper understanding of the complex interplay of genetic variants (e.g., complement factor H, apolipoprotein E) and environmental factors in both diseases is crucial for developing highly personalized and effective therapeutic strategies.
The growing adoption of multidisciplinary teams in CVD management and the increasing recognition of multimorbidity as a major public health concern signify a crucial shift away from a single-disease focus in healthcare. The strong evidence for shared risk factors and, critically, the impact of visual disability on overall CVD risk, naturally lead to the imperative for developing and implementing integrated care models that proactively manage both conditions. Future healthcare systems must evolve to integrate eye care into broader cardiovascular risk assessments, particularly for the rapidly aging population. This entails developing standardized screening protocols that consider both ocular and systemic health, establishing shared electronic health records to facilitate information exchange between specialties, and creating seamless interdisciplinary referral pathways. Such integrated approaches, further refined by precision medicine strategies that tailor interventions to individual patient genetic and lifestyle profiles, are essential to optimize patient outcomes, enhance quality of life, and effectively mitigate the growing burden of co-occurring chronic diseases.
IX. Conclusion
Age-related macular degeneration and cardiovascular disease, though manifesting in distinct organ systems, are deeply intertwined conditions. They share a complex web of common genetic and environmental risk factors, including age, smoking, hypertension, dyslipidemia, obesity, and diabetes. Underlying pathophysiological mechanisms, particularly chronic inflammation, oxidative stress, and systemic vascular dysfunction (including atherosclerosis-like processes observed in the retina), provide a robust biological basis for their frequent co-occurrence. Epidemiological evidence, especially from methodologically sound prospective studies, increasingly supports a significant association, with late-stage AMD and, critically, AMD accompanied by visual disability, linked to an increased risk of overall CVD, heart failure, and all-cause mortality.
The recognition of this profound systemic connection between AMD and CVD necessitates a paradigm shift in clinical practice, moving towards truly integrated management strategies. By acknowledging AMD as a potential indicator of broader systemic vascular health and by aggressively managing shared modifiable risk factors, healthcare professionals can implement "dual prevention" strategies that offer synergistic benefits for both ocular and cardiovascular outcomes. The continued development of multidisciplinary care teams and the advancement of precision medicine approaches hold immense promise for optimizing the health and enhancing the quality of life for an aging global population facing the growing burden of multimorbidity.
X. References
- Hopkins Medicine. Age-Related Macular Degeneration (AMD).
- American Academy of Ophthalmology. Age-Related Macular Degeneration (AMD).
- NHS. Cardiovascular Disease.
- MedlinePlus. Cardiovascular Disease.
- Wang JJ, et al. Data on the association between age-related macular degeneration (AMD) and cardiovascular disease and mortality are conflicting. PLoS One. 2016;11(12):e0166291.
- Wu J, et al. Importance. Research has indicated some shared pathogenic mechanisms between age-related macular degeneration (AMD) and cardiovascular disease (CVD). PLoS One. 2014;9(3):e89600.
- Centers for Disease Control and Prevention. Risk Factors for Heart Disease.
- Klein R, et al. The influences of cardiovascular disease (CVD) (e.g., hypertension, coronary artery disease), CVD risk factors (e.g., total serum cholesterol, body mass index), and CVD treatment (e.g., statins, antihypertensive agents) on AMD are inconsistent across different studies. Invest Ophthalmol Vis Sci. 2009;50(12):5910-5917.
- Harrison D, et al. The endothelium regulates vascular homoeostasis through local elaboration of mediators that modulate vascular tone, platelet adhesion, inflammation, fibrinolysis, and vascular growth. Circ Res. 2003;93(8):759-764.
- Münzel T, et al. Clinical trials have identified chronic inflammatory disorders as cardiovascular risks, and recent research has revealed a contribution by various inflammatory cells to vascular oxidative stress. Antioxid Redox Signal. 2019;31(1):1-30.
- Lim LS, et al. Genetic, epidemiological, and molecular studies are beginning to unravel the intricate mechanisms underlying this complex disease, which implicate the lipid-cholesterol pathway in the pathophysiology of disease development and progression. Prog Retin Eye Res. 2017;57:1-38.
- Shah AD, et al. Cardiovascular multimorbidity (CVM) is the co-occurrence of multiple cardiovascular disease subtypes (CVDs) in one person. Open Heart. 2023;10(2):e002552.
- Cleveland Clinic. Coronary Artery Disease.
- European Heart Journal. Women are underdiagnosed, undertreated and under-represented in clinical trials directed at management strategies for CVD, making their results less applicable to this subset. Heart. 2024;110(22):e3.
- The American Journal of Managed Care. Anemia Linked With Higher Risk of All-Cause, CVD-Related Mortality in Rheumatoid Arthritis. Preventive Medicine Reports. 2025;.
- Tsao CW, et al. We estimate that among adults, prevalence of hypertension will increase from 51.2% in 2020 to 61.0% in 2050. Circulation. 2024;149(1):e3-e18.
- Lee YH, et al. Age‐related macular degeneration (AMD) is the leading cause of visual disability. J Am Heart Assoc. 2023;12(11):e029314.
- Kang S, et al. AMD was associated with a 1.58‐fold increased risk of HF (95% CI, 1.16–1.87) (P<0.001) after adjustment for potential confounders. J Am Heart Assoc. 2021;10(1):e020071.
- The American Journal of Managed Care. 5 Things to Know During AMD Awareness Month.
- Al-Ani A, et al. Current treatments primarily address choroidal neovascularization (CNV) in neovascular AMD. Cells. 2023;12(9):2483.
- Montecucco F, et al. Recent studies have unraveled a more complex and bidirectional relationship between cancer and CVD, suggesting a reverse association of CVD with cancer incidence, progression, and mortality. JACC. 2023;6(7):100067.
- Arnett DK, et al. Clinicians caring for these patients need better guidance on how to mitigate ASCVD progression and major adverse cardiovascular events within the context of other chronic conditions, multiple guideline-directed medical therapies, changing prognoses, and patient preferences for care. J Am Coll Cardiol. 2022;80(16):1598-1632.
- Interes Journals. Comorbidities: Understanding Their Impact on Health and Disease Management.
- Simon TG, et al. Optimising the management of cardiovascular comorbidities in NAFLD patients: it's time to (re-) act! Gut. 2022;71(11):2365-2367.
- Byrne CD, et al. The relationship is two-way with MASLD found in up to 75% of patients with T2DM. Eur J Prev Cardiol. 2025;zwae306..
- Sindi S, et al. Studies link AD with CVD risk factors such as hypertension, inflammation, and dyslipidemia. J Am Heart Assoc. 2023;12(22):e030780.
- Libby P, et al. Randomized controlled trials demonstrated the safety/efficacy of canakinumab and colchicine in secondary cardiovascular prevention, providing confirmation for the involvement of a specific inflammatory pathway (NLRP3 inflammasome/IL [interleukin]-1β) in atherosclerotic Colchicine was recently approved by the US Food and Drug Administration for this indication. Arterioscler Thromb Vasc Biol. 2024;44(6):e3-e18.
- Libby P, et al. Targeting Inflammatory Pathways in Cardiovascular Disease: The Inflammasome, Interleukin-1, Interleukin-6 and Beyond. J Am Coll Cardiol. 2021;77(16):2015-2032.
- Zhang Y, et al. This longitudinal analysis included 271,274 participants who were free of both cardiovascular diseases and AMD at baseline. J Am Heart Assoc. 2024;13(9):e020071.
- Kang S, et al. Heart failure (HF) is a major health problem worldwide because of its high morbidity and mortality. J Am Heart Assoc. 2021;10(1):e020071.
- Al-Ani A, et al. Many medical fields have adopted team-based approaches to decision making due to rapidly evolving and complex treatment algorithms. Cells. 2023;12(9):2483.
- Optimum Health Solutions. Benefits of a multidisciplinary method for Cardiovascular Disease.
- Al-Ani A, et al. These target therapeutic candidates include APE/REF-1, MRZ-99030, Ciliary NeuroTrophic Factor (CNTF), RAP1 GTPase, Celecoxib, and SS-31/Elamipretide. Cells. 2021;10(9):2483.
- Campochiaro PA. While the development of anti-vascular endothelial growth factor (anti-VEGF) as a therapy for neovascular age-related macular degeneration (AMD) was a great success, the pathologic processes underlying dry AMD that eventually leads to photoreceptor dysfunction, death, and vision loss remain elusive to date, with a lack There is an overwhelming need to improve the classification system of AMD, to increase our understanding of cell death mechanisms involved in both neovascular and non-neovascular AMD, and to develop better biomarkers and clinical endpoints to eventually be able to identify better therapeutic targets—especially early in. J Clin Invest. 2016;126(1):89-98.

Leave a comment
This site is protected by hCaptcha and the hCaptcha Privacy Policy and Terms of Service apply.